The Roulette with Conventional "Vaccinations" - PART 17 : Not a single routine childhood vaccine was licensed based on a long-term placebo-controlled trial.
Unfortunately, this article could not be sent via email due to excessive data volume. However, it will soon be linked to in several other articles on this Substack.
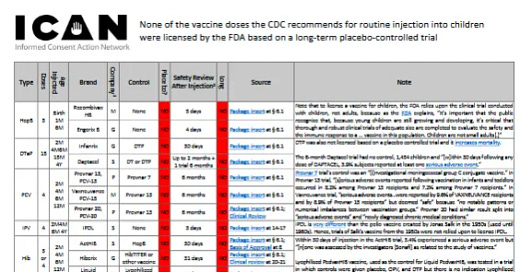
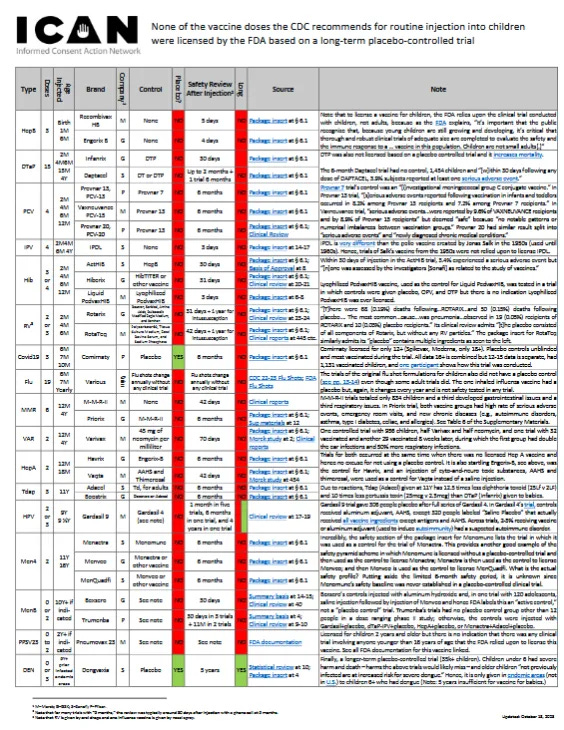
Unfortunately, the lack of placebo controls in paediatric "vaccine" trials is largely unknown. The data in the table at icandecide.org/no-placebo come from the FDA but are nevertheless censored:
https://icandecide.org/wp-content/uploads/2024/03/no-placebo-101823.pdf
The single screenshot is hard to read, but on a split image below you can see everything clearly. The computer magnifying glass helps with the necessary magnification, or please use the link above.
Attorney Aaron Siri devoted a long article to this topic a few years ago. He also listed the individual "vaccines" (a term I've been using in quotes for some time, and for good reason) from the table in his article. Fortunately, there are also links to easily verifiable evidence that can convince anyone interested of the "vaccine" fraud.
Photo : Aaron Siri. He is a lawyer. Here's more information he writes about himself :
Aaron Siri, Managing Partner of Siri & Glimstad, has extensive complex civil litigation experience, including civil rights involving mandated medicine, class actions, and high stakes disputes. www.sirillp.com/aaron-siri/
Source : https://substack.com/@aaronsiri
………………………………………..
What the “Casual Cruelty” of Dr. Paul Offit Reveals
Considered by many to be the world’s leading expert on “vaccine safety”
Aug 04, 2023
https://aaronsiri.substack.com/p/what-the-casual-cruelty-of-dr-paul
“ ( … )
The reality – the hard, clear reality – is that not a single routine childhood vaccine was licensed based on a long-term placebo-controlled trial.
( … )
…………………………………………………………………………………………………………………………
Proof Regarding the Clinical Trials Relied Upon by the FDA to License the Childhood Vaccines on the CDC Childhood Vaccine Schedule
HepB vaccine (Birth 1M 6M)
Recombivax HB (Merck) licensed for babies based on trials with no placebo control & 5 days of safety monitoring after injection. See Package insert § 6.1
Engerix B (GSK) licensed for babies based on trials with no placebo control & 4 days of safety monitoring after injection. See Package insert § 6.1
DTaP vaccine (2M 4M 6M 15M 4Y)
Infanrix (GSK) licensed for babies based on trials with no placebo control (DTP vaccine used as a control) & up to 30 days of safety review after injection. See Package insert § 6.1 (Note that DTP was not licensed in a placebo-controlled trial and increases mortality)
Daptacel (Sanofi) licensed for babies based on trials with no placebo control (DT or DTP vaccine used as control) & 2 months of safety review after injection except one trial which was 6 months with no control, 1,454 children and “[w]ithin 30 days following any dose of DAPTACEL, 3.9% subjects reported at least one serious adverse event.” See Package insert § 6.1 (see note for Infanrix)
PCV vaccine (2M 4M 6M 12M)
Prevnar 13, PCV-13 (Wyeth, part of Pfizer) licensed for babies based on trials with no placebo control (Prevnar 7 used as a control, and Prevnar 7 was licensed based on trial in which the control was another experimental vaccine) & 6 months of safety review after injection which found, “Serious adverse events reported following vaccination in infants and toddlers occurred in 8.2% among Prevnar 13 recipients and 7.2% among Prevnar 7 recipients.” See Package insert § 6.1 (Note the package insert for Prevnar 7 states the control in its licensing trial was an “Investigational meningococcal group C conjugate vaccine”)
Vaxneuvance PCV-15 (Merck) licensed for babies based on trials with no placebo control (Prevnar 13 used as the control) & up to 6 months of safety review after injection finding that, “Among children who received VAXNEUVANCE (N=3,349) or Prevnar 13 (N=1,814) … serious adverse events up to 6 months following vaccination with the 4-dose series were reported by 9.6% of VAXNEUVANCE recipients and by 8.9% of Prevnar 13 recipients.” Deemed “safe” because, “[t]here were no notable patterns or numerical imbalances between vaccination groups.” See Package insert § 6.1
Prevnar 20, PCV-20 (Pfizer) licensed for babies based on trials with no placebo control (Prevnar 13 was used as the control) & up to 6 months of safety review after injection that again showed high rates of serious events (this time broken up into two categories – “serious adverse events (SAEs)” and “newly diagnosed chronic medical conditions (NDCMCs)”) in both vaccine groups but deemed “safe” because “no notable patterns or imbalances between vaccine groups.” See Package insert § 6.1; Clinical Review
Polio vaccine (2M 4M 6M 4Y)
IPOL (Sanofi) licensed in 1990 for babies based on trials with no placebo control & 3 days of safety review after injection. Sanofi reports that, “Although no causal relationship has been established, deaths have occurred in temporal association after vaccination of infants with IPV.” See Package insert at 14-17 (Note that IPOL is an injected polio vaccine and is the only polio vaccine used in the U.S. for over two decades. It is a very different product than the polio vaccine developed by Salk in the 1950s – and, as noted above, ceased being used in the U.S. in the 1960s – and hence the trials of Salk’s vaccine from the early 1950s were not relied upon to license IPOL.)
Hib vaccine (2M 4M 6M 12M)
ActHIB (Sanofi) licensed for babies based on trials with no placebo control (Hepatitis B vaccine used as control) & 30 days of safety review after injection during which 3.4% experienced a serious adverse event but “[n]one was assessed by the investigators [Sonafi] as related to the study of vaccines.” See Package insert § 6.1; Basis of Approval at 8
Hiberix (GSK) licensed for babies based on trials with no placebo control (Unlicensed Hib vaccines and HibTITER used as the control) & 31 days of safety review after injection. See Package insert § 6.1; Clinical review at 20-21
Liquid PedvaxHIB (Merck) licensed for babies based on trials with no placebo control (Lyophilized PedvaxHIB used a control) & 3 days of safety review after injection. See Package insert at 6-8 (Note that Lyophilized PedvaxHIB was tested in a trial in which controls were given placebo, OPV and DTP but there is no indication Lyophilized PedvaxHIB was ever licensed)
Rotavirus vaccine (2M 4M 6M) (Note that every vaccine on the CDC childhood schedule is given via injection, except for one flu vaccine given by nasal spray and the rotavirus vaccines, which are given by oral drops in the mouth.)
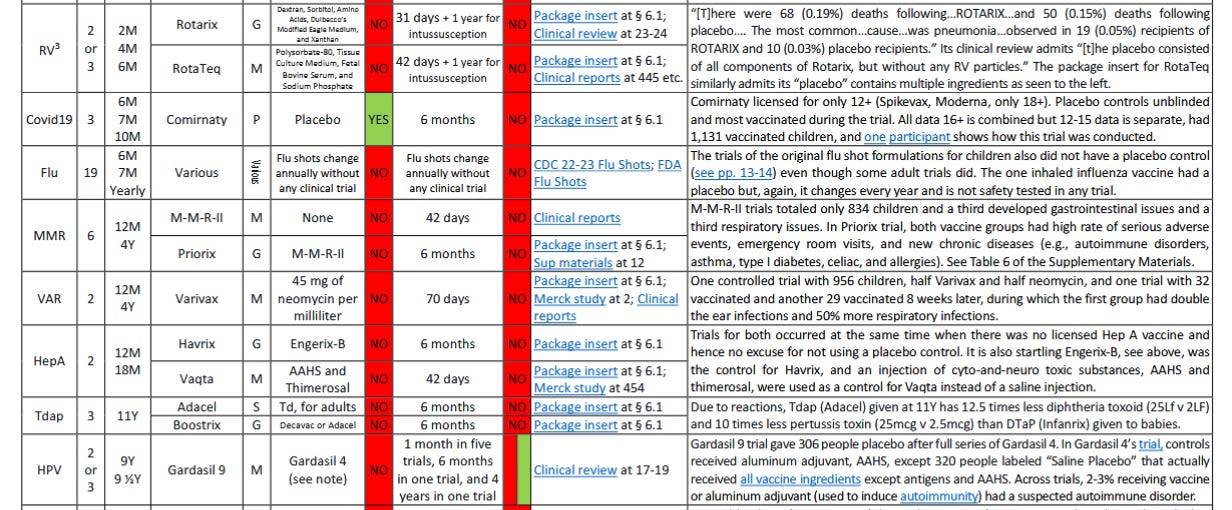
Rotarix (GSK) licensed for babies based on trials without a placebo control (the control group received an oral drop that included Dextran, Sorbitol, Amino Acids, Dulbecco’s Modified Eagle Medium, and Xanthan) & 31 days of safety review after oral dose and up to a year in some trials for cases of intussusception. There were more deaths in the group receiving Rotarix than the purported placebo. As disclosed by the FDA and GSK: “During the entire course of 8 clinical studies (Studies 1 to 8), there were 68 (0.19%) deaths following administration of ROTARIX (n = 36,755) and 50 (0.15%) deaths following placebo administration (n = 34,454). The most commonly reported cause of death following vaccination was pneumonia, which was observed in 19 (0.05%) recipients of ROTARIX and 10 (0.03%) placebo recipients (RR: 1.74, 95% CI: 0.76, 4.23).” See Package insert § 6.1 (claims used a placebo); Clinical review at 23-24 (admits the purported “placebo” included all the foregoing ingredients)
RotaTeq (Merck) licensed for babies based on trials without a placebo control (the control group received an oral drop that included Polysorbate-80, Tissue Culture Medium, Fetal Bovine Serum, and Sodium Phosphate) & 42 days of safety review after each oral dose and up to a year for cases of intussusception. See Package insert § 6.1 (claims used placebo); Clinical reports at 445 etc. (admits the purported “placebo” included all the foregoing ingredients)
Covid-19 vaccine (6M 7M 10M)
Comirnaty (Pfizer) licensed for babies based on trial with a placebo control (finally!) & 6 months of safety review after injection. Package insert § 6.1 (Note that Comirnaty is currently only licensed for 12 years and older and Spikevax, Moderna, is only licensed for 18 years and older. Also, while Comirnaty’s trial had a placebo control group, that group was unblinded and most were vaccinated during the 6-month safety review period. The 16- and 17-year-old data is not separated from the adult data, but the 12- to 15-year-old data is separated and included only 1,131 children who received a vaccine, and the case of one participant reflects how this trial was conducted.)
Flu vaccine (6M 7M Annually)
The formulation for each influenza vaccine changes annually and there is no clinical trial carried out for each new formulation. (In any event, none of the clinical trials for the original formulation of any injected influenza vaccine for children had a placebo control group, see letter pp.13-14, even though some adult trial did, showing it could have been done, see FDA documentation and compare child and adult portions of Section 6.1 of each flu vaccine package insert. The one inhaled influenza vaccine’s original trial had a placebo but, again, its formulation changes every year and is not safety tested in any trial.)
MMR vaccine (12M 4Y)
M-M-R-II (Merck) licensed based on a trial with no placebo control & 42 days of safety review after injection in a trial with a total of only 834 children of which a third developed gastrointestinal issues and a third respiratory issues. See Clinical reports (This patently deficient underpowered, unblinded, and non-randomized trial is unsurprisingly not even listed in the safety section of M-M-R-II’s package insert. Also note that the original MMR’s clinical trial was similarly deficient and also showed a high and concerning rate of gastrointestinal, respiratory and other issues, as compared to the small untreated control group – see pages 12 and 13. In any event, the original MMR was a different product that did not include millions of pieces of human DNA and cellular debris, as does M-M-R-II, which is likely why it was not used as a control in the trial for M-M-R-II).
Priorix (GSK) licensed based on trials with no placebo control (M-M-R-II used as the control) & 6 months of safety review after injection in which both vaccine groups had a high rate of serious adverse events, emergency room visits, and new onset of chronic diseases (e.g., autoimmune disorders, asthma, type I diabetes, vasculitis, celiac disease, thrombocytopenia, and allergies), see second link at page 12. See Package insert § 6.1; Sup materials at 12
Varicella (chicken pox) vaccine (12M 4Y)
Varivax (Merck) licensed based on trials with no placebo control (the purported “placebo” was actually an injection of 45 mg of neomycin per milliliter) & 70 days of safety review after injection which included only one controlled trial of 956 children in which around half received Varivax and half received the injection of 45 mg of neomycin per milliliter, and there was one trial in which 32 children received Varivax and 29 children received nothing and then received Varivax eight weeks later; during this eight-week period, the Varivax group had double the rate of ear infection and a 50% increase in respiratory infection. As for serious adverse events, Merck did not consider any related to Varivax. See Package insert § 6.1; Merck study at 2; Clinical reports
HepA vaccine (12M 18M)
Havrix (GSK) licensed based on trials with no placebo control (Engerix-B was used as a control) & 31 days of safety review after injection with a phone call follow-up at 6 months. Package insert § 6.1
Vaqta (Merck) licensed based on trials with no placebo control (an injection of AAHS, an aluminum adjuvant, and thimerosal, a form of mercury, were used as a control) & up to 42 days of safety review after injection. Package insert § 6.1 (using term “placebo”); Merck study at 454 (admits the purported “placebo” included all the foregoing ingredients) (Note that trials for Havrix and Vaqta occurred at roughly the same time and, because there was no licensed Hepatitis A vaccine at that time, there was no excuse for not using a placebo control in these trials. It is also startling that Engerix-B, which had 4 days of safety monitoring in its trial, was used as the control for Havrix, and that an injection of known cyto-and-neuro toxic substances, AAHS and thimerosal, were used as a control for Vaqta instead of just a saline injection.)
Tdap vaccine (11Y)
Adacel (Sanofi) licensed based on trials with no placebo control (Td, for adult use, was used as a control) & up to 6 months of safety review after injection. See Package insert § 6.1
Boostrix (GSK) licensed based on trials with no placebo control (DECAVAC or Adacel was used as a control) & up to 6 months of safety review after injection. See Package insert § 6.1
HPV vaccine (9Y 9 ½Y)
Gardasil 9 (Merck) was licensed based on trials in which safety was reviewed after injection for 1 month in five of the clinical trials, 6 months in a lot consistency trial, and 4 years in one trial of women aged 16 to 26 years (reflecting that a safety trial of a more appropriate duration is possible). These Gardasil 9 trials were either not controlled or used Gardasil 4 as the control except for one trial in which 306 participants received a placebo but only after receiving the full series of Gardasil 4 injections. See Clinical review at 17-19 (Note that in Gardasil 4’s clinical trial, controls received aluminum adjuvant, AAHS, except 320 people labeled “Saline Placebo” that actually received all vaccine ingredients except antigens and AAHS. Also, across all these trials, 2-3% of participants receiving vaccine or aluminum adjuvant -- used to induce autoimmunity -- had a suspected autoimmune disorder.)
Men4 vaccine (11Y 16Y)
Menactra (Sanofi) licensed based on trials with no placebo control (Menomune used as the control, and amazingly the safety section of the package insert for Menomune lists this same trial in which it is being used as a control) & up to 6 months of safety review after injection. See Package insert § 6.1
Menveo (GSK) licensed based on trials with no placebo control (Menactra, Boostrix, or other vaccines used as a control) & up to 6 months of safety review after injection. Package insert § 6.1
MenQuadfi (Sanofi) licensed based on trials with no placebo control (Menveo or other vaccines used as a control) & up to 6 months of safety review after injection. Package insert § 6.1 (The three Men4 vaccines provides another good example of the vaccine safety pyramid scheme because Menomune was licensed without a placebo-controlled trial and then used as the control to license Menactra; Menactra is then used as the control to license Menveo; and then Menveo is used as the control to license MenQuadfi. The actual safety profile, putting aside the limited 6-month safety period, is unknown since Menomune’s safety baseline was never established in a placebo-controlled trial.)
MenB vaccine (10Y+ if indicated)
Bexsero (GSK) licensed based on trials with no placebo control group (either uncontrolled or control group was given an injection of aluminum hydroxide and, in one trial involving 120 adolescents, a saline injection followed by an injection of Menveo and hence FDA labels this an “active control” and not a “placebo control” trial) & 30 days of safety review after injection. See Summary basis at 14-15; Clinical review at 40
Trumenba (Pfizer) licensed based on trials with no placebo control group other than 12 people in a dose ranging phase II study (otherwise the controls were injection of Gardasil+placebo, dTaP-IPV+placebo, HepA+placebo, or Menactra+Adacel+placebo) & 30 days of safety review after injection for one of the three trials and up to 11 months in the other two trials. See Summary basis at 4; Clinical review at 9-10
PPSV23 vaccine (2Y+ if indicated)
Pneumovax 23 (Merck) is licensed for children 2 years and older but there is no indication that there was any clinical trial involving anyone younger than 16 years of age that the FDA relied upon to license this vaccine. See FDA documentation
Dengue vaccine (6Y+ if previously had dengue and live in area dengue is endemic)
Dengvaxia (Sanofi) licensed based on a trial with 11,474 children receiving a placebo control (saline injection) & 5 years of safety review after injection. Meaning, the 17th and last vaccine on the CDC’s childhood vaccine schedule, is apparently the first vaccine that underwent a longer-term placebo-controlled trial prior to licensure! This trial stands as the proof that a longer-term placebo-controlled trial of a childhood vaccine is possible! See Statistical review at 10; Package insert at 4 (Note for this vaccine, it was learned that children under 6 years old had an increased risk of severe harm and death from this vaccine – harm that would likely never be uncovered by the trials performed for any of the other 16 vaccines. It was also found that children older than 6 who had never had dengue and received this vaccine likewise had a seriously increased risk of severe harm and death. Hence, this vaccine is only to be given to older children who have previously had dengue. As disclosed by the FDA and Sanofi: “Those not previously infected are at increased risk for severe dengue disease when vaccinated and subsequently infected with dengue virus.” This vaccine is only recommended for children in endemic dengue areas and dengue is not endemic in the United States.)
Full article :
https://aaronsiri.substack.com/p/what-the-casual-cruelty-of-dr-paul
…………………………………………………………………………………………………………………………
………………………………………………………………………………………………………………………….
The most reliable information on the “Covid” hoax and the deceptions of the system can be found in the statements of Dr. Mike Yeadon, at the links below :
Dr. Mike Yeadon's Substack #1 :
https://drmikeyeadon.substack.com/
( & https://substack.com/@drmikeyeadon )
The Telegram channel of Dr. Mike Yeadon ( other Telegram channels with his name are fake ! ) :
https://t.me/DrMikeYeadonsolochannel
A collaborative Substack by Dr. Yeadon and Suavek ( Dr. Mike Yeadon's Substack #2 ) :
Fraud Prevention Hotline / suavek1.substack.com
DEAR FRIENDS,
The two Substacks, Dr. Yeadon's and Suavek's, have merged into a single, highly informative entity. The Fraud Prevention Hotline is now officially Dr. Yeadon's Substack No. 2. You can find his statement on this at the following link :
https://drmikeyeadon.substack.com/p/my-other-substack
We urge you, if possible, to add both Substacks to your recommended list in your Substack. Thank you very much in advance,
Mike & Suavek
………………………………
The possible support goes to Suavek. I would like to express my sincere thanks to the 27 people who have supported my work so far with 5 euros per month or 50 euros per year.
You can either do something against or for something :