Own research ? Online access to reports of suspected side effects. Also important in relation to veterinary medicine and mRNA-bioweapons.
EudraVigilance - European database of suspected adverse drug reaction reports.
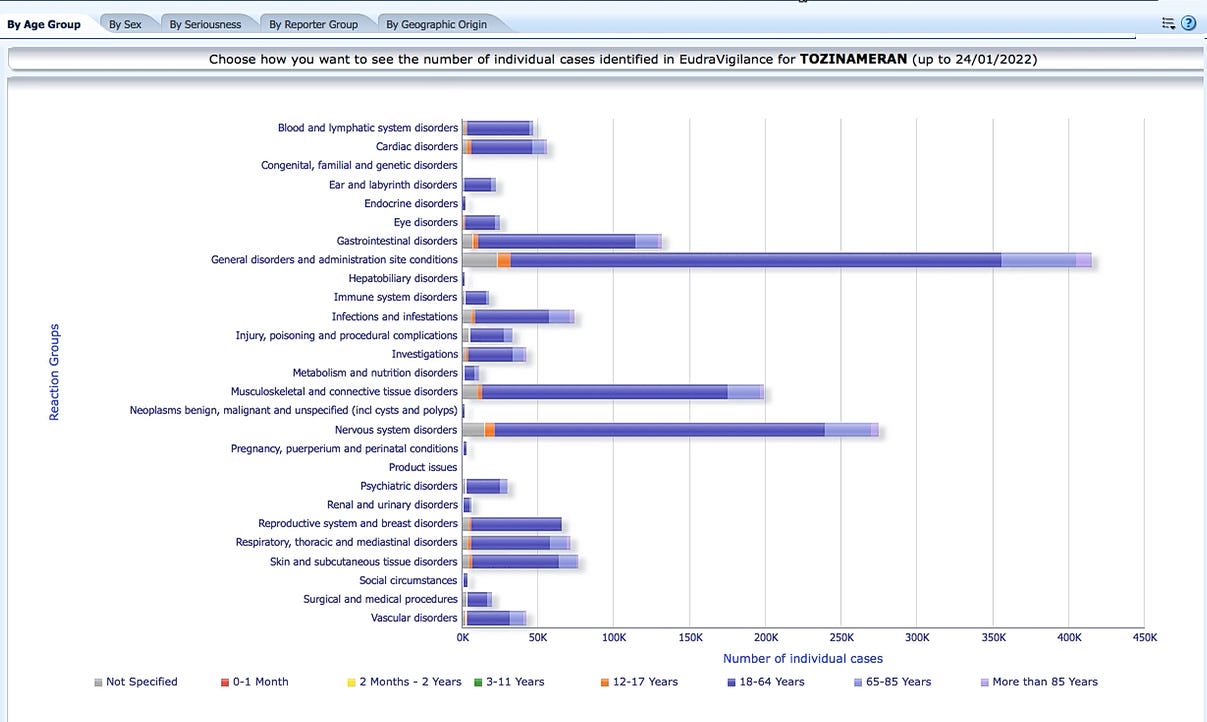
The most important data seems to be somewhat hidden on this website. This is how the idea for this article was born: the time-consuming search should be simplified.
………………………………………………………………………………………………………………………….
EudraVigilance - European database of suspected adverse drug reaction reports.
Online access to suspected side-effect reports
Note :
This is data coming from one of the most corrupt institutions of all time. It is strongly recommended that you make your own corrections to the numbers. Typically, only between 1 and 10 percent of adverse side effects are reported. There have been cases in the health authorities where thousands of reported numbers simply disappeared from the websites overnight, given strange excuses and absurd reasons.
………………………………………………………………………………………………………………………….
The first steps in the search / the operating instructions :
EudraVigilance - European database of suspected adverse drug reaction reports ( Belongs to : European Medicines Agency ( EMA ) :
https://www.adrreports.eu/en/search_subst.html#
or :
You can choose the language at the top right.
First, the cookies must be confirmed (below) :
and then click "Search" ( above ) :
Only then can you continue with the next steps.
If you are looking for medication then select :
“Suspected adverse drug reaction reports for Products”
The selected button appears in black :
Select the appropriate letter for the medication below. For the Covid “vaccinations” that would be the letter “C” :
A fragment of the list. Select the bioweapon you are looking for here :
As an example : A video created by a Polish user :
…………………………………………………………………………………………………………………………
Important for veterinarians :
https://dap.ema.europa.eu/analytics/saw.dll?PortalPages
………………………………………………………………………………………………………………………….
General search for different types of medical products :
https://www.ema.europa.eu/en/medicines
………………………………………………………………………………………………………………………….
Conflicts of interest and corruption at the European Medicines Agency
…………………………………………………………………………………………
Inquiry to : European Medicines Agency
( Request under the Freedom of Information Act )
The text has been partially translated
Dear Ema and / or EU,
many know that it is a fact that the EU/EMA has lots of "conflicts of interest"
It is even said the American medical industry is jelous of the European situation. It is open knowledge that directors of EMA were/are involved with "big pharma". So I guess I do not need to quote many sources for this, but here is a newer report on the issue:
"Conflits d’intérêt et corruption à l’Agence européenne du médicament"
Source: "France Soir"
"Conflicts of Interest and Corruption at the European Medicines Agency"
Sources: "Liberty Beacon" and "Europe Reloaded"
My request:
Any and all documents/data/explainations etc. how this can be possible and how the EU/EMA safeguards that there is still some "neutral" decission making in effect. Also documents about how much € the EMA gets from the pharma and/or chemical industries (here in the years 2018,2019 and 2020).
Pls. provide every document related to above issues, I am ok with the redaction of personal info and prefer eg. PDF or Word documents.
Thanks
…………………………………………………………………………………………………………..
The response from European Medicines Agency :
…………………………………………………………………………………………………………..
European Medicines Agency
Dear Mr Antragsteller/in , Thank you for your recent request for information. We can provide you with the following information on your 2 questions **Any and all documents/data/explainations etc. how this can be possible and how the EU/EMA safeguards that there is still some "neutral" decission making in effect.** Independence of Management Board members, committee members and experts and EMA staff is safeguarded by strict conflict of interest policies and high level of transparency. EMA’s policies on handling competing interests ([ https://www.ema.europa.eu/en/about-us... ( https://www.ema.europa.eu/en/about-us... )]) have been put in place to restrict the involvement of Management Board members, committee members and experts and EMA staff with possible competing interests in the Agency’s work while maintaining EMA’s ability to access the best available expertise. We would invite you to check out these documents that contain further information on EMA’s measures to safeguard independence. Please note that Management Board members, members and experts of EMA’s committees, working parties and scientific advisory groups or ad hoc expert groups and EMA staff members submit a declaration of interests prior to any involvement in EMA activities or recruitment. The declarations of interests of all Management Board members, committee members and experts who take part in EMA activities, and EMA senior management are published on the EMA website ( https://www.ema.europa.eu/en ). EMA also publishes annual reports (2020 report: https://www.ema.europa.eu/en/document... ( https://www.ema.europa.eu/en/document... )) on its independence, which include facts and figures on declared interests and resulting restrictions. **Also documents about how much € the EMA gets from the pharma and/or chemical industries (here in the years 2018,2019 and 2020).** As you may already know, European legislation requires that pharmaceutical companies contribute to the costs of regulation of medicines. As the companies will earn revenues from the sales of medicines, it is fair that they should bear most of the financial costs of regulating them. Companies pay an administrative fee upfront before EMA assessment starts. The administrative fee applicable for each procedure is defined by EU legislation. We would like to refer youto this web page [ https://www.ema.europa.eu/en/about-us... ( https://www.ema.europa.eu/en/about-us... )] where you can find several information regarding the financial and budgetary reports from the European Medicines Agency. More specifically section 4.5 at page 25 of the Agency’s annual accounts 2020 provides a breakdown of the fees by major categories ([ https://www.ema.europa.eu/en/document... ( https://www.ema.europa.eu/en/document... )]). A similar report is available for the year 2019 ([ https://www.ema.europa.eu/en/document... ( https://www.ema.europa.eu/en/document... )] ) and 2018 ([ https://www.ema.europa.eu/en/document... ( https://www.ema.europa.eu/en/document... )]). We hope that this information is useful for you. Best regards, **European Medicines Agency** Domenico Scarlattilaan 6, 1083 HS Amsterdam, The Netherlands Send us a question. Go to www.ema.europa.eu/contact ( http::// www.ema.europa.eu/contact ) Telephone: +31 (0)88 781 6000 _________________________________________________We received your question(s) on: **04/01/2022** Subject of your enquiry: **(AskEMA-FragdenStaat) Conflicts of interest and corruption at the European Medicines Agency [#236573]** Your question(s): **Antrag nach EU-Verordnungen 1049/2001 sowie 1367/2006 Sehr geehrte Damen und Herren, auf Basis der Verordnungen 1049/2001 sowie 1367/2006 bitte ich Sie um Übersendung von Dokumenten, die folgende Informationen enthalten: Dear Ema and / or EU, many know that it is a fact that the EU/EMA has lots of "conflicts of interest" It is even said the American medical industry is jelous of the European situation. It is open knowledge that directors of EMA were/are involved with "big pharma". So I guess I do not need to quote many sources for this, but here is a newer report on the issue: "Conflits d’intérêt et corruption à l’Agence européenne du médicament" Source: "France Soir" "Conflicts of Interest and Corruption at the European Medicines Agency" Sources: "Liberty Beacon" and "Europe Reloaded" My request: Any and all documents/data/explainations etc. how this can be possible and how the EU/EMA safeguards that there is still some "neutral" decission making in effect. Also documents about how much € the EMA gets from the pharma and/or chemical industries (here in the years 2018,2019 and 2020). Pls. provide every document related to above issues, I am ok with the redaction of personal info and prefer eg. PDF or Word documents.
Source :
Other links to corruption reports:
https://www.europereloaded.com/massive-fraud-in-reporting-vaccine-injuries-withheld-data/
https://www.francesoir.fr/politique/ursula-et-heiko-von-der-leyen-conflits-d-interets-au-minimum
………………………………………………………………………………………………………………………..
Check your batch:
Batch codes and associated deaths, disabilities and illnesses for Covid 19 Vaccines
👉 https://howbadismybatch.com/
…………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………….
Would you like to support my enlightenment work with a coffee ?
With your donating you are supporting my enlightenment work for thousands peoples. This is very much appreciated !
Thank you !
.................................................................
My Telegram channel : Join 👉 https://t.me/QueueForBrain
……………………………………………………………………………………………………………………...