Recognize fraudulent studies and manufacturer claims yourself-PART 1: The work of Prof. Norman Fenton, Prof. Martin Neil, and Dr. Toby Rogers as a detection aid.
One of the examples: The use of toxic placebos instead of saline solution.
Foreword :
by Suavek
Examining scientific studies for signs of fraud has become a favourite pastime of many doctors, scientists, and laypeople since 2020. The relevant literature on the "vaccine" fraud has existed as long as the fraud itself, but unfortunately, only now have the authors of these books been taken seriously. This article is intended to help every layperson identify inconsistencies when reading studies and manufacturer information. The easiest way is to determine which information is missing and to know which topics are completely unregulated by law, thus leaving the industry's fraudsters free rein. Of course, every loophole will be exploited. We all pay the price for this, and our children with our own health.
As for the work of Toby Rogers, excerpts from which appear at the end of the article, it should be noted that the topic relevant here only begins with Chapter 9. The relevant text can be found a little further down in this article. This is because I've also compiled an index of the various chapters of his work, so you know what other topics are covered here and can click on the original source if necessary. That's why you'll find so many symbols like this one: "(...)". However, in Chapter 9, you'll find some indirect clues in the form of examples of what to look for in your exam. While the number of examples is limited, the series of articles in this Substack has only just begun with this Part 1. The next parts will follow.
One more tip: On the Substack by Prof. Norman Fenton and Prof. Martin Neil, "Where are the numbers?" ( https://wherearethenumbers.substack.com/ ), you'll often find articles that denounce the inconsistencies in the studies. Norman Fenton's website may also be helpful : https://www.normanfenton.com/ . In the "Publications" column, you'll find the subcategory "Covid-19": https://www.normanfenton.com/covid-19. Here are some articles pointing out inconsistencies in various studies. Unfortunately, the articles don't directly clarify that the disease "Covid" never existed, which should increase the chances of publication. Professors Norman Fenton and Martin Neil have indirectly and directly exposed so many "Covid" lies and related frauds that it must be clear to everyone that the edifice of lies has long since collapsed. Both, of course, know full well that we were never dealing with an "extraordinary, new health threat."
…………………………………………………………………………………………………………………………
A few examples of the article by Norman Fenton and Martin Neil :
……………………………..
Photo : left - Prof. Norman Fenton, right - Prof. Martin Neil.
Cheap Trick paper rejected by 'Vaccines' journal
Peer review successfully avoided!
Martin Neil, Norman Fenton, and Dr Scott McLachlan
Apr 11, 2025
We submitted the latest version of our ‘cheap trick’ paper ( Editor's note: The full article is now unfortunately behind a pay bar ) to Vaccines - the international, peer-reviewed, open access journal, hoping they would be open minded and brave enough to review and hopefully accept it for publication.
We received this response a few days later:
We regret to inform you that we will not be processing your submission further. Submissions sent for peer-review are selected based on discipline, novelty and general significance, in addition to the usual criteria for publication in scholarly journals. Therefore, our decision does not necessarily reflect the quality of your work.
We wish you every success if you choose to pursue publication elsewhere.
Kind regards,
Vaccines Editorial Office
vaccines@mdpi.com
It didn’t even go out to review but was rejected - instantly!
( … )
So much for peer review!
( … )
Full article :
https://wherearethenumbers.substack.co
m/p/cheap-trick-paper-rejected-by-vaccines
…………………………………………………………………………………………………………………………
Can we trust ‘independent peer review’?
May 20, 2025
“( … )
Editor's note: The points below were part of a sworn statement by Prof. Norman Fenton:
1. It is important for the tribunal to note that ‘peer review in reputable journals’ is a misunderstood and overrated concept in scientific publishing. I have some experience of peer review having published some 400 peer reviewed articles during my 48-year career and been a peer reviewer of many hundreds. I have also been on the editorial board of many journals. Peer review increasingly is not a measure of quality and accuracy nor an indication of validity. The following points are an assessment of how corrupt and meaningless peer review has become, especially in medical research. Few academics would publicly admit this as it would not serve their careers to do so.
2. First of all, there is little ‘independence’ in peer review. Many areas of academic research are highly specialised, often giving rise to cliques within those areas which typically revolve around a handful of established professors, their former and current PhD students and research assistants. These professors get appointed to editorial boards of journals and so can control what gets published. Papers in subjects relevant to their specialism from those who are part of their clique will be sent to review by other members of their clique.
( … )
4. ( … ) As most reputable journals require at least 3 reviewers, it is much easier to get people you know to review papers. In fact, the more specialised the area the more likely only people in that clique will be competent to review papers in that area. In my experience I would say that it is rare for more than 1 in 10 reviews to be thorough – most are just cursory. In the most specialised areas, there will be a consensus view of what constitutes the ‘correct approach’. ( … ).
5. Because of the difficulty of finding reviewers, many journals now invite authors to recommend reviewers. ( … ).
6. There are also powerful non-academic interests which dictate what research is acceptable and which cliques can dominate editorial boards of the top tier journals. In the case of medical publishing those conflicts of interests involve the pharmaceutical industry and charitable foundations.
7. A recent paper[1] exposed the extent to which pharma companies are essentially paying reviewers. The effect of this on the quality and integrity of ‘peer reviewed’ research has been devastating. Professors Heneghan and Jefferson from Oxford University’s Centre for Evidence Based Medicine describe how broken the peer review system is.
8. The corruption of the peer review process reached new levels during the covid pandemic. In our book we catalogue the extent to which most well researched articles that challenge the ‘official narrative’ of ‘a deadly virus defeated by a safe and effective vaccine’ have been censored by the major journals. They rarely even get passed on to review by the Editors. Even preprint servers often refuse to publish them on the basis that they provide claims that ‘contradict WHO recommendations’. At the same time flawed or superficial papers that support the narrative – often without providing access to the data on which studies are based – are accepted. When ‘sceptics’ write letters (or papers) rebutting these flawed papers they are rarely answered and never printed. The Lancet has been one of the worst journals playing these tricks as chronicled by my own example when I pointed out the flaws in one of the most cited papers on vaccine efficacy. On some of the extremely rare occasions when papers seriously challenging the narrative get published (typically in less prestigious journals) they have been withdrawn after the most frivolous complaints.
( … ).”
Full article :
https://wherearethenumbers.substack.com/p/can-we-trust-independent-peer-review
…………………………………………………………………………………………………………………………
Most peer reviewers for major medical journals receive industry payments. You can read about it in this article:
… or view the study directly here:
…………………………………………………………………………
Research Letter
Payments by Drug and Medical Device Manufacturers to US Peer Reviewers of Major Medical Journals
Published Online: October 10, 2024. doi:10.1001/jama.2024.17681
David-Dan Nguyen, MDCM, MPH1,2; Anju Murayama3,4; Anna-Lisa Nguyen, BHSc5; et al
Source : https://jamanetwork.com/journals/jama/fullarticle/2824834
“ ( … )
Methods
We identified peer reviewers for The BMJ, JAMA, The Lancet, and The New England Journal of Medicine (NEJM) using each journal’s 2022 reviewer list. These journals were selected for their high impact factor and reputation as leading publications of original general medical research. ( … ).
We extracted general and research payments to the identified peer reviewers between 2020 and 2022 from the Open Payments database, capturing payments from drug and medical device manufacturers to US-licensed physicians.4 We excluded ownership and investment interests because they are not equivalent to financial transfers and are less reliable than other general payments. Research payments included payments to individual physicians and institutional payments for research where they served as principal investigators. Institutional payments were divided by the number of principal investigators. Inflation-adjusted payment amounts in 2022 US dollars were calculated among those receiving payments.
( … )
Results
Among 7021 reviewer names, including duplicates, we excluded 332 reviewers who were not searchable in Scopus, 3257 non-US reviewers, and 1325 nonphysicians. This left 1962 unique reviewers, of whom 145 (7.4%) had performed peer reviews for more than 1 journal.
Between 2020 and 2022, 1155 peer reviewers (58.9%) received at least 1 industry payment (Table 1).
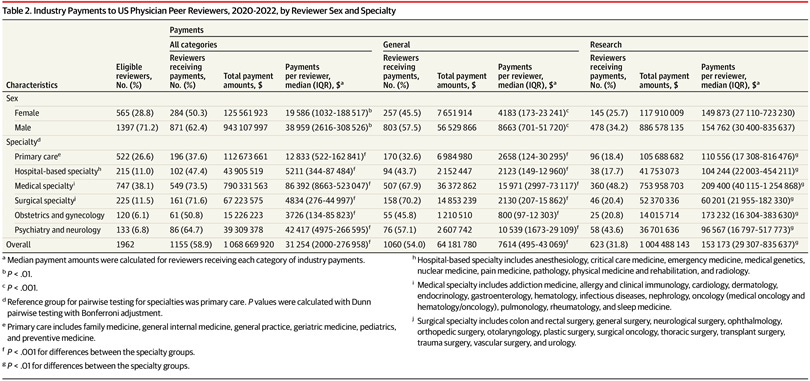
Table 1 :
More than half (54.0%) of reviewers accepted general payments, while 31.8% received research payments.
Reviewers received $1.06 billion in industry payments between 2020 and 2022, including $1.00 billion (94.0%) to individuals or their institutions and $64.18 million (6.0%) in general payments. Consulting fees and speaking compensation unrelated to continuing medical education programs accounted for $34.31 million and $11.80 million, respectively. Over the 3 years, the median general payment was $7614 (IQR, $495-$43 069) and the median research payment was $153 173 (IQR, $29 307-$835 637) among reviewers receiving such payments.
Male reviewers had significantly higher median total payments ($38 959 vs $19 586) and general payments ($8663 vs $4183) than female reviewers. Statistically significant differences in payments existed between specialties (Table 2).
Table 2 :
Discussion
More than half of the 1962 US physicians included in this study who peer reviewed for the most influential medical journals received industry payments in 2020-2022, with most payments for research. Research payments, especially those provided to an institution, may have different implications than general payments for conflicts of interest. Peer reviewers in this study received $64.18 million in general payments between 2020 and 2022, representing a median general payment of $7614, larger than the median general payment to all physicians in 2018 of $216.5
( … ).”
Full article :
https://jamanetwork.com/journals/jama/fullarticle/2824834
………………………………………………………………………………………………………………………….
The Political Economy of Autism
Toby Rogers
A thesis submitted to fulfil the requirements for the degree of Doctor of
Philosophy, Department of Political Economy, School of Social and
Political Sciences, Faculty of Arts and Social Sciences, University of
Sydney, 2019.
Editor's note: This paper was published in 2019, so some information here is considered outdated. The index of individual chapters is provided only to indicate what other topics you can read by clicking on the original source. Scrolling down will take you to Chapter 9, which contains information relevant to this article.
( … )
Acknowledgements
( … )
Abstract
( … )
List of abbreviations and acronyms
( … )
Styles
( … )
Table of Contents
( … )
Glossary
( … )
Part I:
Background and context
( … )
Chapter 1
History, prevalence, cost, and theories of causation of ASD
( … )
Chapter 2
Theoretical framework
( … )
Chapter 3
What can the histories of science and medicine teach us about
governments’ failure to engage in autism prevention?
( … )
Chapter 4
Case studies in toxic chemical disasters and
long term toxic chemical exposures
( … )
Chapter 5
The capitalist conquest of science and medicine
( … )
Chapter 6
The failure to investigate possible environmental factors in general and
the failure to regulate endocrine disruptors in particular in the U.S.
( … )
Chapter 7
The failure to effectively regulate herbicides and pesticides
( … )
Chapter 8
The failure to effectively regulate SSRIs
( … )
Chapter 9
The political economy of the regulation of vaccines
9.0 Introduction to the vaccines and autism debate
( … )
9.1.0 Introduction to the political economy of vaccines
( … )
9.1.1 The size of the vaccine market
( … )
9.1.2 The growth in the U.S. vaccine schedule
In 1983, the official U.S. vaccine schedule could be explained in half a page and consisted of the following vaccines: DTP (diphtheria, tetanus, and pertussis) (2 months), OPV (oral polio vaccine) (2 months), DTP (4 months), OPV (4 months), DTP (6 months), MMR (measles, mumps, and rubella) (15 months), DTP (18 months), OPV (18 months), DTP (4 years), OPV (4 years), and Td (tetanus and diphtheria without pertussis) (15 years) (CDC, 1983). So, counting each component of the conjugate vaccines separately, the schedule contained 24 doses total consisting of 7 injected vaccines and 4 oral vaccines. In 1985 the autism rate in the U.S. was calculated at 1 in 2,500 (Autism Speaks, 2010). The current vaccine schedule (CDC, 2017) takes eight pages to explain and consists of the following:
DTaP (diphtheria, tetanus, and acellular pertussis); Hib (haemophilus influenzae type b); HPV (human papillomavirus); IPV (inactivated poliovirus); MMR (measles, mumps, rubella); PCV (pneumococcal conjugate vaccine); Tdap (tetanus, diphtheria, and acellular pertussis) — which is different than DTaP.
As one can see the schedule is heavily front-loaded into the first two years of life with as many as seven injections at the six month paediatric ‘well baby’ visit. In total, counting each component of the conjugate vaccines separately, the current schedule has 74 doses of 53 injected vaccines and three oral vaccines. Presently the autism rate is estimated at 1 in 36 (Zablotsky et al., 2017). This simply shows temporal association, but the association is troubling and that is one of the cases to answer here.
9.1.3 The vaccine approval process in the U.S.
( … )
9.1.4 Vaccine licensure process at the FDA is characterised by studies that are short, small, and often use toxic placebos
Vaccine safety studies used in the licensure process are conducted by the manufacturers which presents a clear conflict of interest. In this subsection I will present evidence that vaccine safety studies are too short and often use toxic placebos instead of saline in order to hide adverse reactions.
f hypothetically, iatrogenic injury from vaccines had a long latency period — because of the toxicokinetics of the vaccine or the dynamics of autoimmune disease or the difficulties created by the fact that the target market are infants who are unable to narrate their own internal experience — many of the vaccine safety studies that have been approved by the FDA would not have been able to identify them because the trials were so short. For example, there are two hepatitis B vaccines licensed for use in the U.S. — one made by Merck and one made by GlaxoSmithKline. The evidence suggests neither of them has been adequately tested for safety in the target population. Holland (2012) notes that the package insert for Merck’s Recombivax HB states: ‘In three clinical studies, 434 doses of Recombivax HB, 5 mcg, were administered to 147 healthy infants and children (up to 10 years of age) who were monitored for 5 days after each dose’ (p. 70). Holland (2012) points out that ‘the insert does not state the ages of the children or the proportion of the 147 subjects who were infants. It makes no mention of newborns’ even though it is now given to almost all newborns (p. 70). By the same token, the package insert for GlaxoSmithKline’s (GSK) Engerix-B states: ‘In 36 clinical studies, a total of 13,495 doses of Engerix-B were administered to 5,071 healthy adults and children who were initially seronegative for hepatitis B markers, and healthy neonates. All subjects were monitored for 4 days post-administration’ (Holland, 2012, p. 70). There is no mention of how many neonates were in the study and the study results were not broken out separately for this population either (Holland, 2012, p. 70).
But it is not just the hepatitis B vaccine. Relatively short safety studies are the norm in the licensing of vaccines. The package insert for the Hib (haemophilus influenzae type b) vaccine manufactured by Merck shows that it was licensed based on a trial that monitored adverse reactions for three days; the package insert of the Hib vaccine manufactured by GSK shows that it was licensed based on a trial that monitored adverse reactions for four days; and ‘the only stand-alone polio vaccine was licensed after a mere 48-hour follow-up period’ (Informed Consent Action Network, 2017b, p. 2).
In addition to short safety studies, vaccines are almost always tested against toxic placebos rather than saline in order to hide adverse events. As I showed in chapter 5, Contract Research Organisations (CROs) have a number of ways that they can produce the outcomes desired by their pharmaceutical company clients. CROs are under enormous pressure to maximise the evidence of efficacy and minimise the evidence of harms. One way that many vaccine studies are manipulated is that instead of using a saline placebo as part of an RCT, another vaccine is used as the comparator instead. Alternatively, CROs put the toxic adjuvants (ethylmercury, aluminium) in the placebo and the only difference between the drug and placebo is the biological agent. None of this is prohibited by law. Beatrice Golomb (1995) then at the Department of Medicine at UCLA drew attention to problems with placebos in a letter to Nature:
The U.S. Food and Drug Administration sets no regulation on the constituents of placebos, and any guidelines are at best informal. Astonishingly, no systematic efforts are made to ensure the inertness of placebos: there is nothing validating the placebo standard against which other agents are measured. Further, the drug companies funding the trials control the placebo ingredients. The identity of the placebo and fillers used with the experimental drug are rarely stated in scientific studies (p. 530).
Fifteen years later, Golomb et al. (2010) showed that there are still no regulations concerning the contents of placebos in the U.S. In a review of all RCTs published in the four highest impact medical journals in 2008 and 2009, only 34% of studies of injectable treatments fully disclosed the contents of the placebo (Golomb et al., 2010, p. W-189). Jacobson, Ovsyannikova, and Poland (2009) reviewed four recent vaccine trials involving children and none of them used a saline placebo. ICAN (2018) reviewed the FDA applications for all of the vaccines currently on the national schedule and discovered that none of them used saline placebos (with the exception of Gardasil-9 [HPV vaccine] where Merck ran a small subtrial using a saline placebo — but all of the study participants had previously received three doses of the original Gardasil vaccine and were in good health) (p. 6).
9.1.5 Conflicts of interest at the VRBPAC and ACIP
( … )
9.1.6 Post-market surveillance of vaccine adverse events is inadequate
( … )
9.1.7 Revolving door between CDC and vaccine makers
( … )
9.1.8 Lobbying at the state level to make vaccines compulsory
( … )
9.1.9 COI in scientific journals associated with vaccines
( … )
9.2.0 Introduction to the scientific debate on vaccine safety
( … )
9.2.1 What are the net benefits to society from widescale vaccination campaigns?
( … )
9.2.2 How important is herd immunity?
( … )
9.2.3 Is ethylmercury toxic (at the doses contained in the vaccine schedule)?
( … )
9.2.4 Is aluminium toxic (at the doses contained in the vaccine schedule)?
( … )
9.2.5 Are other vaccine ingredients toxic (at the doses contained in the vaccine schedule)?
( … )
9.2.6 Studies showing no association between vaccines and autism
( … )
9.2.7 Vaccinated vs. unvaccinated studies
In the absence of government funded double blind RCTs on the safety of the vaccine schedule (or even prospective or retrospective comparisons between vaccinated and unvaccinated groups), many vaccine safety and autism advocacy groups have funded their own surveys and studies. As I pointed out in chapter 1, this is an example of what Moore (2006) calls Activist-Initiated Participatory Science and in some cases these surveys and studies might be considered part of what Rose (2017) calls the ‘Autism Literary Underground’.
Are these studies valid? It is hard to know. The same issues of the funding effect apply in these cases as with the corporate and government funded studies — any COI no matter how small has been shown to change research outcomes. Most of these are conflicted studies so there is reason for scepticism. The situation is made considerably more complicated by the fact that mainstream public health institutions refuse to conduct vaccines safety studies with an unvaccinated control group — so there are no studies that contradict these findings. It makes for an untenable situation — on the one hand a number of studies by advocacy groups show startling results; on the other hand mainstream actors claim that these results are unlikely to be true and then refuse to conduct the sort of studies that would resolve the matter. But there are two studies that are not characterised by a financial conflict of interest (Gallagher & Goodman, 2008 and 2010) and their results are in line with the findings of the other vaccinated vs. unvaccinated studies reviewed in this section.
( … )
Table 9.1: Clinical Data from Integrative Pediatrics
Source: Thomas and Margulis (2016), p. 326.
Given current estimates of 1 in 36 children with ASD, one would expect to see about 30 cases in the group of children vaccinated according to the alternative schedule and about seven cases in the unvaccinated population — instead there were none; while the 1/60 autism cases for children vaccinated according to the CDC schedule is just slightly lower than the national average (Thomas & Margulis, 2016, p. 327). Are these figures valid? Thomas has a financial conflict of interest so scepticism is warranted and these results have not been published in a peer reviewed journal. But if the figures are correct, it would mean that a big piece of the autism puzzle may have been found. Given the stakes, one would think public health officials and media outlets would take an immediate interest in following up to verify whether the data are correct. As of this writing (September 2018), there was no sign of follow up from the CDC or any other federal agency and none of the top 10 (by circulation) newspapers in the country have reviewed the book.
Gallagher and Goodman (2008 and 2010) are also vaccinated vs. unvaccinated studies and I will review them in the subsection below.
9.2.8 Hepatitis B vaccine
( … )
9.2.9 Troubling recent data on the benefits and safety of the flu vaccine
( … )
9.2.10 Allegations of fraud in vaccine safety research
( … )
9.2.11 Vaccines do not work very well in the first year of life
( … )
Editor's note: This paper was written in 2019. I firmly believe that today, in 2025, the author knows that there are no vaccines at all that can produce a positive effect. He is an avid reader of Dr Mike Yeadon's statements, and this Substack.
9.3 Lobbying and campaign contributions by vaccine makers
( … )
9.4 Conclusion
( … )
Chapter 10
Conclusion
( … )
References
( … )
Full article :
https://ses.library.usyd.edu.au/bitstream/handle/2123/20198/Rogers_T_thesis.pdf
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
The most reliable information on the “Covid” hoax and the deceptions of the system can be found in the statements of Dr. Mike Yeadon, at the links below :
Dr. Mike Yeadon's Substack #1 :
https://drmikeyeadon.substack.com/
( & https://substack.com/@drmikeyeadon )
The Telegram channel of Dr. Mike Yeadon ( other Telegram channels with his name are fake ! ) :
https://t.me/DrMikeYeadonsolochannel
A collaborative Substack by Dr. Yeadon and Suavek ( Dr. Mike Yeadon's Substack #2 ) :
Fraud Prevention Hotline / suavek1.substack.com
DEAR FRIENDS,
The two Substacks, Dr. Yeadon's and Suavek's, have merged into a single, highly informative entity. The Fraud Prevention Hotline is now officially Dr. Yeadon's Substack No. 2. You can find his statement on this at the following link :
https://drmikeyeadon.substack.com/p/my-other-substack
We urge you, if possible, to add both Substacks to your recommended list in your Substack. Thank you very much in advance,
Mike & Suavek
………………………………
The possible support goes to Suavek. I would like to express my sincere thanks to the 27 people who have supported my work so far with 5 euros per month or 50 euros per year.
You can either do something against or for something :












Valuable information thank you for compiling!